anti infliximab elisa kit|ab237659 anti : vendor Anti-Ly6g antibody [1A8] - mouse IgG2c (Chimeric) . Note: Infliximab ELISA kit ab237647 is intended for Research Use Only, not for use in diagnostic procedures. This kit is a sandwich based ELISA kit. The color developed is proportional to the amount of Infliximab in the sample or standard. Results of samples can be determined directly using . We created this 17-question eBook as a framework to help you explore and discover the exact type of autoclave best suited to your needs. These questions will help you .
{plog:ftitle_list}
Un autoclave es un recipiente de presión metálico de paredes gruesas con un cierre hermético que permite trabajar a alta presión para una cocción o una esterilización con See more
anti
is graduated pipette more accurate than volumetric
ab237659 anti
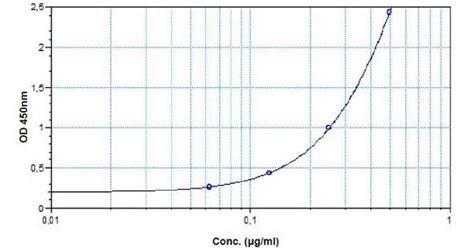
anti-Infliximab ELISA Kit is a Sandwich (quantitative) ELISA kit for the measurement of anti-Infliximab in Human in Plasma, Serum samples.The anti-infliximab ELISA Kit (ab237659) is a highly specific and sensitive kit designed for the in vitro determination of the antibody against Infliximab in biological matrices such as human .BioVision's BioSimTM anti-Infliximab ELISA kit is designed to quantify/measure the antibody against Infliximab with high specificity and sensitivity in biological .
Anti-Ly6g antibody [1A8] - mouse IgG2c (Chimeric) . Note: Infliximab ELISA kit ab237647 is intended for Research Use Only, not for use in diagnostic procedures. This kit is a sandwich based ELISA kit. The color developed is proportional to the amount of Infliximab in the sample or standard. Results of samples can be determined directly using .Three commercially available assays for the measurement of antibodies to infliximab (ATI) are approved for clinical use in Australia: Promonitor anti-infliximab (Grifols), Lisa Tracker anti-infliximab (Theradiag) and Ridascreen anti-IFX (R-Biopharm). All are bridging ELISA assays. Measurement of ATI .Anti-Infliximab ELISA Kit (DEIABL214), manufactured by Creative Diagnostics with high sensitivity and reproducibility, providing efficient and accurate result for lab research.

Anti-Infliximab ELISA KBI2011, Ver2.0 www.eaglebio.com 1 REF Anti-Infliximab ELISA : KBI2011 Ver 2.0 RUO Enzyme Immunoassay for the quantitative determination of Anti- . This ELISA kit detects antibodies for Anti-Infliximab and may be used for . The incidence of anti-infliximab antibodies ranges widely from 6.1 to 73% and nearly 35% for ADL . A . Finally, antibody detection using Promonitor ELISA kits is a robust method with high sensitivity and specificity, but, as with its drug testing counterpart, multiple samples need to be accumulated to make it cost-effective. .
is graduated pipette qualitative
ab237656 anti-Infliximab ELISA Kit 1 1. Overview Anti-Infliximab ELISA Kit (ab237656) is designed to quantify/measure the antibody against Infliximab with high specificity and sensitivity in biological matrices. Infliximab is a therapeutic chimeric monoclonal antibody against tumor necrosis factor (TNF) and is used to treat rheumatic arthritis,Infliximab ELISA kits are assays designed to detect the chimeric monoclonal IgG1 antibody infliximab. It is composed of constant human and variable murine regions. It works by binding and neutralizing TNF-α, a potent proinflammatory cytokine.The anti-infliximab ELISA Kit (ab237659) is a highly specific and sensitive kit designed for the in vitro determination of the antibody against Infliximab in biological matrices such as human serum and plasma. Infliximab (CT-P13) is a chimeric monoclonal antibody and used toThe Infliximab (Remicade®) ELISA kit can be used for monitoring Infliximab during clinical trials and research, by measuring free Infliximab in a serum. . Anti- Infliximab is coated onto a 96 well microplate. Calibrator, quality control samples (if desired) and test samples are pipetted into the appropriate wells. Infliximab present in .
Intended use:RIDASCREEN® IFX Monitoring is an enzyme linked immunoassay intended for the quantitative determination of infliximab (IFX, Remicade®, anti-TNFα) and its biosimilars Remsima®, Inflectra® and Flixabi® in human serum and plasma. Key features of RIDASCREEN® IFX Monitoring: validated in clinical trials highly specific monoclonal antibody MA-IFX6B7, .Quantitative ELISA kits for anti-drug antibodies (immunogenicity) with confirmation reagent available for 18 different biological drugs. Matriks Biotek® is the first biotechnology company which produced ELISA kits for Remsima® (Infliximab Biosimilar CT-P13). Kits for Remsima® includes quantitative free drug detection kits, qualitative Anti .Quantify Rituximab in human serum/plasma with KRIBIOLISA ELISA Kit. Utilizing a precise immunoassay for accurate results in clinical research. . a HRP (horseradish peroxidase) conjugated anti-Infliximab antibody is pipetted and incubated. After washing microwells in order to remove any non-specific binding, the ready to use substrate solution .
Agreement among methods for measurement of anti-infliximab antibodies. The agreement was good only for the comparison between the two ELISA kits (82.5 %; κ = 0.65), with 7 samples providing positive anti-IFX antibodies in LT ELISA but negative ones according to .
Anti-Infliximab Free Drug/ADA Dual ELISA Kit . Enzyme-linked immunosorbent assay for the semi-quantitative determination of total and free antibodies to infliximab in serum and plasma. Free and Total Anti-Drug Antibody (ADA) monitoring gained high importance along with measuring free drug from the patient samples. In order to make these .
to Infliximab ELISA Kit has been designed for the measurement of free antibodies against this drug. It also detects such antibodies which already are bound to the . During sample preparation, the Infliximab-bound anti-Infliximab antibodies are dissociated from the complex in order to acquire free anti-Infliximab antibodies. During the .
Total Infliximab ELISA Assay Kit Catalog Number: IG-TA101 (1 x 96 wells) For Research Use Only. Not for use in diagnostic procedures. v. 1.0 . preparation, the Infliximab-bound anti-Infliximab antibodies are dissociated from the complex in order to acquire free antiInfliximab antibodies. During the incubation period, antibodies to
Infliximab Antibody ELISA Kit for Measurement of Chemical, Human Infliximab Antibody in Plasma (EDTA - heparin), Serum etc. English +1 877 302 8632; Contact . One of the major concern, despite of its wide usage, is the potential development of anti-Infliximab antibodies (ATI) which in turn may interfere with Infliximab efficacy as mainly .Anti-Infliximab-Ab (ATI) ELISA Enzyme immunoassay for the quantitative determination of antibodies to infliximab in human serum and plasma QS51101 96 For illustrative purposes only. To perform the assay the instructions for use provided with the kit have to be used. Distributed by: I B L I N T E R N A T I O N A L G M B H Anti-drug-antibodies (ADA) against infliximab are frequently measured in patients receiving infliximab treatment with loss of response and undetectable infliximab concentrations. Different ADA bridging assays (1st generation, 2nd generation and ready-to-use kit) have been developed successively and were applied over the last 10 years, making comparison between . CHORUS Promonitor INFLIXIMAB kit was an ELISA quantitative assay, CHORUS Promonitor ADALIMUMAB was a sandwich ELISA, whereas CHORUS Promonitor ANTI-INFLIXIMAB and CHORUS Promonitor ANTI-ADALIMUMAB kits were semiquantitative assays based on bridging ELISA. . CHORUS Promonitor ANTI-INFLIXIMAB kit was linear from 2.3 .
Background: There are many studies presenting data of biologics and several ELISA kits commercially available for monitoring infliximab serum trough levels (s-IFXt) and anti-drug antibodies (ADAb). We propose to compare technical characteristics and results of three different assays on a cohort of 35 patients under infliximab (IFX) and suffering from inflammatory bowel .
The Promonitor®-anti-IFX kit is used for semi-quantitative determination of anti-IFX antibodies. This ELISA assay quantifies anti-IFX antibody levels. The procedure is similar to the previous one, except the signal obtained is proportional to the amount of .Fiorino G, Ruiz-Argüello MB, Maguregui A, et al. Full interchangeability in regard to immunogenicity between the infliximab reference biologic and biosimilars CT-P13 and SB2 in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(3):601-606. Product Detail Specification; Method. Quantitative bridging ELISA. Microplate size. 96 wells. Kit .Infliximab and etanercept caused false positives in Lisa-Tracker and Shikari kits. Anti-ADM antibody ELISA kits performed differently with spiked samples because of different measuring units and ranges. Ridascreen and Shikari kits were dose responsive across the entire standard curve and correlated well with each other (r = 0.997).
Kit Insert Request Quote. Availability: 3 – 4 Weeks | Pack Size: 1 x 96 wells. KRIBIOLISA™ Anti-Infliximab (REMICADE) ELISA quantity. Add to Cart. . The KRIBIOLISA Anti-Infliximab (Remicade) ELISA is used as an analytical tool for quantitative determination of Anti-Infliximab antibodies in serum, plasma and cell culture supernatant. .Anti-Infliximab (Human) antibodies ELISA Kit. Catalog Number: IEK-02-1158. Size: 1 plate (96 wells) Anti-Infliximab ELISA Kit is a sandwich enzyme-linked immunoassay for the quantitative determination of Anti-Infliximab with high sensitivity and specificity in serum and plasma.
Anti-Infliximab antibody ELISA Kit Alias Tumor necrosis factor ELISA Kit, Cachectin ELISA Kit, TNF-alpha ELISA Kit, TNF ELISA Kit, TNFA ELISA Kit, TNFSF2 ELISA Kit Catalogue No. EU3590 Size 48T/96T Species Universal UniProt No. P01375 Sample Type Serum, Plasma, Cell Culture Supernatant, cell or tissue lysate, Other liquid samples
The new Pod-8 and Pod-12 Autoclaves have been designed to specifically meet the needs of podiatrists and foot health professionals. They benefit from a compact footprint and user .A PodoCenter é uma empresa séria e inovadora que tem como objetivo atender tanto os profissionais das áreas de Podologia e Estética, quanto consumidores finais.
anti infliximab elisa kit|ab237659 anti